1. Tranexamic acid evidence and controversies: An illustrated review
TXA ILLUSTRATED REVIEW
Tranexamic acid evidence and controversies: An illustrated review
Nicole Relke MD,Nicholas L. J. Chornenki MD,Michelle Sholzberg MDCM, MSc, FRCPC
First published: 14 July 2021 https://doi.org/10.1002/rth2.12546Citations: 3
Handling Editor: Alisa Wolberg.
Tranexamic acid evidence and controversies: An illustrated review
Nicole Relke MD,Nicholas L. J. Chornenki MD,Michelle Sholzberg MDCM, MSc, FRCPC
First published: 14 July 2021 https://doi.org/10.1002/rth2.12546Citations: 3
Handling Editor: Alisa Wolberg.
ACKNOWLEDGMENTS
Select images in this review were taken from https://smart.servier.com and are licensed under a Creative Commons Attribution 3.0 Unported License. Blood drop image (pages 1, 4-5, 9-11) was purchased from shutterstock (stock vector ID: 641207020)
RELATIONSHIP DISCLOSURE
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
NR and MS conceived and designed the manuscript. NR, NLJC, and MS wrote the paper.
REFERENCES
1Watts G. Utako Okamoto (Obituary). Lancet. 2016; 387: 2286.
CrossrefPubMedWeb of Science®Google Scholar
2Okamoto S, Okamoto U. Amino-methyl-cyclohexane-carboxylic acid: AMCHA. A new potent inhibitor of fibrinolysis. Keio J Med. 1962; 11: 105- 115.
CrossrefCASGoogle Scholar
3Olldashi F, Kerçi M, Zhurda T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010; 376: 23- 32.
CrossrefPubMedWeb of Science®Google Scholar
4 WHO Expert Committee. The selection and use of essential medicines (including the 17th WHO model list of essential medicines). WHO Tech Rep Ser [Internet]. 2011; 2011: 1- 249.
Google Scholar
5Shakur H, Roberts I, Fawole B, et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017; 389: 2105- 2116.
CrossrefCASPubMedWeb of Science®Google Scholar
6Thibault GE. Women in academic medicine. Acad Med. 2016; 91: 1045- 1046.
CrossrefPubMedWeb of Science®Google Scholar
7Mannucci PM. Hemostatic drugs. NEJM. 1998; 339; 245- 253.
CrossrefCASPubMedWeb of Science®Google Scholar
8Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015; 29: 17- 24.
CrossrefCASPubMedWeb of Science®Google Scholar
9Lin H, Xu L, Yu S, Hong W, Huang M, Xu P. Therapeutics targeting the fibrinolytic system. Exp Mol Med. 2020; 52: 367- 379.
CrossrefCASPubMedWeb of Science®Google Scholar
10Linkins LA, Takach LS. Review of D-dimer testing: Good, Bad, and Ugly. Int J Lab Hematol. 2017; 39: 98- 103.
Wiley Online LibraryPubMedWeb of Science®Google Scholar
11Johnson ED, Schell JC, Rodgers GM. The D-dimer assay. Am J Hematol. 2019; 94: 833- 839.
Wiley Online LibraryPubMedWeb of Science®Google Scholar
12Tengborn L, Blombäck M, Berntorp E. Tranexamic acid - an old drug still going strong and making a revival. Thromb Res. 2015; 135: 231- 242.
CrossrefCASPubMedWeb of Science®Google Scholar
13Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336: 924- 926.
CrossrefPubMedWeb of Science®Google Scholar
14Novikova N, Hofmeyr GJ, Cluver C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2015; 1- 62.
Web of Science®Google Scholar
15Myles PS, Smith JA, Forbes A, et al. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017; 376: 136- 148.
CrossrefCASPubMedWeb of Science®Google Scholar
16 Collaborators C-3 trial. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019; 394: 1713- 1723.
CrossrefPubMedWeb of Science®Google Scholar
17Ac B, Lethaby A, Farquhar C, Hickey M. Antifibrinolytics for heavy menstrual bleeding (Review). Cochrane Database Syst Rev. 2018; 1- 76.
Web of Science®Google Scholar
18La S, Warner GT, Cope E. Treatment of menorrhagia with tranexamic acid. a double-blind trial summary: a double-blind trial. Br Med J. 1970; 4: 214- 216.
CrossrefPubMedGoogle Scholar
19Lukes A, Moore K, Muse K, Gersten J. Tranexamic acid treatment for heavy menstrual bleeding. J Obstet Gynecol. 2010; 116: 865- 867.
CrossrefGoogle Scholar
20Sprigg N, Flaherty K, Appleton JP, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. The Lancet. 2018; 391: 2107- 2115. https://doi.org/10.1016/S0140-6736(18)31033-X
CrossrefCASPubMedWeb of Science®Google Scholar
21Wand O, Guber E, Guber A, Epstein Shochet G, Israeli-Shani L, Shitrit D. Inhaled tranexamic acid for hemoptysis treatment. Chest. 2018; 154: 1379- 1384. https://doi.org/10.1016/j.chest.2018.09.026
CrossrefPubMedWeb of Science®Google Scholar
22Calvo GS, De Granda-Orive I, Padilla DL. Inhaled tranexamic acid as an alternative for hemoptysis treatment. Chest. 2016; 149: 604. https://doi.org/10.1016/j.chest.2015.10.016
CrossrefPubMedWeb of Science®Google Scholar
23Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009; 123: 687- 696.
CrossrefCASPubMedWeb of Science®Google Scholar
24Wang Z, Zhang H. Comparative effectiveness and safety of tranexamic acid plus diluted epinephrine to control blood loss during total hip arthroplasty: a meta-analysis. J Orthop Surg Res. 2018; 13: 1- 11.
CrossrefPubMedWeb of Science®Google Scholar
25Morimoto Y, Yoshioka A, Sugimoto M, Imai Y, Kirita T. Haemostatic management of intraoral bleeding in patients with von Willebrand disease. Oral Dis. 2005; 11: 243- 248.
Wiley Online LibraryCASPubMedWeb of Science®Google Scholar
26Eghbali A, Melikof L, Taherahmadi H, Bagheri B. Efficacy of tranexamic acid for the prevention of bleeding in patients with von Willebrand disease and Glanzmann thrombasthenia: a controlled, before and after trial. Haemophilia. 2016; 22: e423- e426.
Wiley Online LibraryCASPubMedWeb of Science®Google Scholar
27Montroy J, Hutton B, Moodley P, et al. The efficacy and safety of topical tranexamic acid: a systematic review and meta-analysis. Transfus Med Rev. 2018; 32: 165- 178.
CrossrefWeb of Science®Google Scholar
28Zahed R, Mousavi Jazayeri MH, Naderi A, Naderpour Z, Saeedi M. Topical tranexamic acid compared with anterior nasal packing for treatment of epistaxis in patients taking antiplatelet drugs: randomized controlled trial. Acad Emerg Med. 2018; 25: 261- 266.
Wiley Online LibraryPubMedWeb of Science®Google Scholar
29Joseph J, Martinez-Devesa P, Bellorini J, Burton MJ. Tranexamic acid for patients with nasal haemorrhage (epistaxis). Cochrane Database Syst Rev. 2018; 2018: 1- 38.
Google Scholar
30Geisthoff UW, Seyfert UT, Kübler M, Bieg B, Plinkert PK, König J. Treatment of epistaxis in hereditary hemorrhagic telangiectasia with tranexamic acid - a double-blind placebo-controlled cross-over phase IIIB study. Thromb Res. 2014; 134: 565- 571.
CrossrefCASPubMedWeb of Science®Google Scholar
31Shpilberg O, Blumenthal R, Sofer O, et al. A controlled trial of tranexamic acid therapy for the reduction of bleeding during treatment of acute myeloid leukemia. Leuk Lymphoma. 1995; 19: 141- 144.
CrossrefCASPubMedWeb of Science®Google Scholar
32Avvisati G, Büller HR, Ten CJW, Mandelli F. Tranexamic acid for control of haemorrhage in acute promyelocytic leukaemia. Lancet. 1989; 334: 122- 124.
CrossrefGoogle Scholar
33Kim HJ, Moon SH, Cho SH, Lee JD, Kim HS. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017; 97: 776- 781.
CrossrefCASPubMedWeb of Science®Google Scholar
34Compels MM, Lock RJ. C1 inhibitor deficiency: management. Clin Exp Dermatol. 2005; 30: 737- 740.
Wiley Online LibraryPubMedWeb of Science®Google Scholar
35Sentilhes L, Winer N, Azria E, et al. Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med. 2018; 379: 731- 742.
CrossrefCASPubMedWeb of Science®Google Scholar
36Sentilhes L, Daniel V, Deneux-Tharaux C. TRAAP2-TRAnexamic Acid for Preventing postpartum hemorrhage after cesarean delivery: a multicenter randomized, doubleblind, placebo-controlled trial-a study protocol. BMC Pregnancy Childbirth. 2020; 20: 1- 11.
CrossrefWeb of Science®Google Scholar
37Ker K, Roberts I, Chaudhri R, et al. Tranexamic acid for the prevention of postpartum bleeding in women with anaemia: study protocol for an international, randomised, double-blind, placebo-controlled trial. Trials. 2018; 19: 1- 19.
CrossrefPubMedWeb of Science®Google Scholar
38 Pfizer Canada. Product Monograph: Cyklokapron [Internet]; 2018. https://www.pfizer.ca/cyklokapron-tranexamic-acid (accessed December 16, 2020).
Google Scholar
39 Tranexamic acid. FDA. [Internet]; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212020lbl.pdf (accessed January 25, 2021).
Google Scholar
40Muse K, Lukes AS, Gersten J, Waldbaum A, Mabey RG, Trott E. Long-term evaluation of safety and health-related quality of life in women with heavy menstrual bleeding treated with oral tranexamic acid. Women’s Heal. 2011; 7: 699- 707.
CrossrefCASPubMedGoogle Scholar
41Lecker I, Wang DS, Whissell PD, Avramescu S, Mazer CD, Orser BA. Tranexamic acid-associated seizures: causes and treatment. Ann Neurol. 2016; 79: 18- 26.
Wiley Online LibraryCASPubMedWeb of Science®Google Scholar
42Gofton TE, Chu MWA, Norton L, et al. A prospective observational study of seizures after cardiac surgery using continuous EEG monitoring. Neurocrit Care. 2014; 21: 220- 227.
CrossrefPubMedWeb of Science®Google Scholar
43Manji RA, Grocott HP, Leake J, et al. Seizures following cardiac surgery: the impact of tranexamic acid and other risk factors. Can J Anesth. 2012; 59: 6- 13.
CrossrefPubMedWeb of Science®Google Scholar
44Kalavrouziotis D, Voisine P, Mohammadi S, Dionne S, Dagenais F. High-dose tranexamic acid is an independent predictor of early seizure after cardiopulmonary bypass. Ann Thorac Surg [Internet]. 2012; 93: 148- 154. https://doi.org/10.1016/j.athoracsur.2011.07.085
CrossrefPubMedWeb of Science®Google Scholar
45Lin Z, Xiaoyi Z. Tranexamic acid-associated seizures: a meta-analysis. Seizure [Internet]. 2016; 36: 70- 73. https://doi.org/10.1016/j.seizure.2016.02.011
CrossrefPubMedWeb of Science®Google Scholar
46Baharoglu MI, Germans MR, Rinkel GJE, et al. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2013; 2013.
Google Scholar
47Imbesi S, Nettis E, Minciullo PL, et al. Hypersensitivity to tranexamic acid: a wide spectrum of adverse reactions. Pharm World Sci. 2010; 32: 416- 419.
CrossrefCASPubMedWeb of Science®Google Scholar
48Li PH, Trigg C, Rutkowski R, Rutkowski K. Anaphylaxis to tranexamic acid—a rare reaction to a common drug. J Allergy Clin Immunol Pract [Internet]. 2017; 5: 839- 841. https://doi.org/10.1016/j.jaip.2016.12.014
CrossrefPubMedWeb of Science®Google Scholar
49Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012; 43: 1711- 1737.
CrossrefPubMedWeb of Science®Google Scholar
50Post R, Germans MR, Tjerkstra MA, et al. Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet. 2020; 6736: 1- 7.
Google Scholar
51Hallberg L, Högdahl AM, Nilsson L, Rybo G. Menstrual blood loss—a population study. Variation at different ages and attempts to define normality. Obstet Gynecol Surv. 1967; 22: 653- 654.
CrossrefGoogle Scholar
52 Heavy menstrual bleeding clinical guideline 2007. National Collaborating Centre for Women’s and Children’s Health. NICE; 2007.
Google Scholar
53Connell NT, Flood VH, Brignardello-Petersen R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 2021; 5: 301- 325.
CrossrefCASPubMedWeb of Science®Google Scholar
54Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009; 145: 212- 220.
Wiley Online LibraryCASPubMedWeb of Science®Google Scholar
55Roberts I, Shakur-Still H, Afolabi A, et al. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet. 2020; 395: 1927- 1936.
CrossrefPubMedWeb of Science®Google Scholar
56Roberts LN. Tranexamic acid in acute gastrointestinal bleeding – a cautionary tale. J Thromb Haemost. 2020; 18: 2440- 2443.
Wiley Online LibraryCASPubMedWeb of Science®Google Scholar
57Blasi A, Patel VC, Adelmeijer J, et al. Mixed fibrinolytic phenotypes in decompensated cirrhosis and acute-on-chronic liver failure with hypofibrinolysis in those with complications and poor survival. Hepatology. 2020; 71: 1381- 1390.
Wiley Online LibraryCASPubMedWeb of Science®Google Scholar
58Lisman T, De Groot PG, Meijers JCM, Rosendaal FR. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005; 105: 1102- 1105.
CrossrefCASPubMedWeb of Science®Google Scholar
59Gernsheimer T, Brown SP, Triulzi DJ, et al. Effects of tranexamic acid prophylaxis on bleeding outcomes in hematologic malignancy: the a-TREAT trial. Blood. 2020; 136: 1- 2.
CrossrefPubMedWeb of Science®Google Scholar
60Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47: 207- 214.
Wiley Online LibraryCASPubMedWeb of Science®Google Scholar
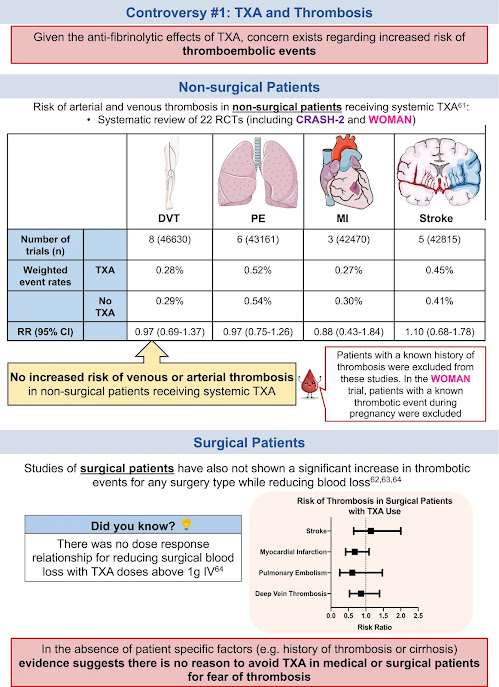
61Chornenki NLJ, Um KJ, Mendoza PA, et al. Risk of venous and arterial thrombosis in non-surgical patients receiving systemic tranexamic acid: a systematic review and meta-analysis. Thromb Res [Internet]. 2019; 179: 81- 86. https://doi.org/10.1016/j.thromres.2019.05.003
CrossrefCASPubMedWeb of Science®Google Scholar
62Taeuber I, Weibel S, Herrmann E, et al. Association of intravenous tranexamic acid with thromboembolic events and mortality. A systematic review, meta-analysis, and meta-regression . JAMA Surg. 2021; 156; 1- 14.
CrossrefWeb of Science®Google Scholar
63Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012; 344: 1- 13.
CrossrefGoogle Scholar
64Ker K, Prieto-Merino D, Roberts I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013; 100: 1271- 1279.
Wiley Online LibraryCASPubMedWeb of Science®Google Scholar
65Thorne JG, James PD, Reid RL. Heavy menstrual bleeding: is tranexamic acid a safe adjunct to combined hormonal contraception? Contraception [Internet]. 2018; 98: 1- 3. https://doi.org/10.1016/j.contraception.2018.02.008
CrossrefCASPubMedWeb of Science®Google Scholar
66Heinemann LAJ, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007; 75: 328- 336.
CrossrefPubMedWeb of Science®Google Scholar
67Reid RL, Westhoff C, Mansour D, et al. Oral contraceptives and venous thromboembolism: consensus opinion from an international workshop held in Berlin, Germany in December 2009. J Fam Plan Reprod Heal Care. 2010; 36: 117- 122.
CrossrefPubMedWeb of Science®Google Scholar
68Iacobellis G, Iacobellis G. Combined treatment with tranexamic acid and oral contraceptive pill causes coronary ulcerated plaque and acute myocardial infarction. Cardiovasc Drugs Ther. 2004; 18: 239- 240.
CrossrefCASPubMedWeb of Science®Google Scholar
69Klok FA, Schreiber K, Stach K, et al. Oral contraception and menstrual bleeding during treatment of venous thromboembolism: expert opinion versus current practice. Thromb Res. 2017; 153: 101- 107.
CrossrefCASPubMedWeb of Science®Google Scholar
70Meschino D, Sholzberg M. Evaluating the combined effect of anti-fibrinolytics and estrogen on the risk of thromboembolism: a scoping review. Available from: osf.io/edj28
Google Scholar
71Vujkovac B, Sabovic M. A successful treatment of life-threatening bleeding from polycystic kidneys with antifibrinolytic agent tranexamic acid. Blood Coagul Fibrinolysis. 2006; 17: 589- 591.
CrossrefPubMedWeb of Science®Google Scholar
72AlAmeel T, West M. Tranexamic acid treatment of life-threatening hematuria in polycystic kidney disease. Int J Nephrol. 2011; 2011: 1- 3.
CrossrefGoogle Scholar
73Peces R, Aguilar A, Vega C. Medical therapy with tranexamic acid in autosomal dominant polycystic kidney disease patients with severe haematuria. Nefrologia. 2012; 32: 160- 165.
PubMedWeb of Science®Google Scholar
74Lee S, Fralick M, Sholzberg M, et al. Systematic-review of Hematuria and Acute Renal-failure with cycloKapron (SHARK); 2020.
Google Scholar
Citing Literature
Number of times cited according to CrossRef: 3
Stephanie G. Lee, John Fralick, Christopher J. D. Wallis, Monica Boctor, Michelle Sholzberg, Michael Fralick, Systematic review of hematuria and acute renal failure with tranexamic acid, European Journal of Haematology, 10.1111/ejh.13762, 108, 6, (510-517), (2022).
Wiley Online Library
Sarah Nersesian, Michelle Sholzberg, Mary Cushman, Alisa S. Wolberg, The Journey to a Successful Illustrated Review, Research and Practice in Thrombosis and Haemostasis, 10.1002/rth2.12721, 6, 4, (2022).
Wiley Online Library
Giuliana D’Aulerio, Nagendra N. Dudi‐Venkata, Chris Varghese, Uyen G. Vo, Peter Pockney, Toby Richards, David I. Watson, Deborah Wright, Doug Robb, Liam Ferguson, Jana‐Lee Moss, William Xu, Bridget Hwang, Laure Taher Mansour, Warren Seow, Scott Gelzinnis, Joel Seto, Cameron I. Wells, José Antonio García‐Erce, James Glasbey, Sivesh K. Kamarajah, Kenneth McLean, Ane Abad Motos, Francesco Pata, Gianluca Pellino, Javier Ripolles, Postoperative variations in anaemia treatment and transfusions (POSTVenTT): protocol for a prospective multicentre observational cohort study of anaemia after major abdominal surgery, Colorectal Disease, 10.1111/codi.15902, 24, 2, (228-234), (2021).
Wiley Online Library
Por favor compartir nuestras REDES SOCIALES @DrRamonReyesMD, así podremos llegar a mas personas y estos se beneficiarán de la disponibilidad de estos documentos, pdf, e-book, gratuitos y legales..
https://www.instagram.com/drramonreyesmd/
https://www.pinterest.es/DrRamonReyesMD/
https://www.linkedin.com/in/drramonreyes/
https://twitter.com/eeiispain
Blog
http://emssolutionsint.blogspot.com/2016/12/dr-ramon-reyes-diaz-md-emt-t-dmo.html
TELEGRAM
Group https://t.me/joinchat/GRsTvEHYjNLP8yc6gPXQ9Q




%20paint.heic)







No comments:
Post a Comment