 |
| Trillon de microbios puede vivir en 1gr de CACA "Lavese las Manos despues de ir al Baño" |
Mantener las manos limpias es una de las mejores formas de prevenir la propagación de infecciones y enfermedades.
El lavado de manos es algo fácil de hacer y es una de las formas más eficaces de prevenir la propagación de muchos tipos de infecciones y enfermedades en todos los lugares, desde su casa* y el sitio de trabajo,* hasta las guarderías infantiles y los hospitales.* Las manos limpias pueden evitar que los microbios pasen de una persona a otra y a toda la comunidad.
http://emssolutionsint.blogspot.com/2020/05/covid-19-geles-y-soluciones.html
Sepa más sobre cuándo y cómo lavarse las manos.
¿Cuándo debe lavarse las manos?

- Antes, durante y después de preparar alimentos.
- Antes de comer.
- Antes y después de atender a alguien que esté enfermo.
- Antes y después de tratar heridas o cortaduras.
- Después de usar el baño.
- Después de cambiar pañales o limpiar a un niño que haya ido al baño.
- Después de sonarse la nariz, toser o estornudar.
- Después de haber tocado animales, alimento para animales o excrementos de animales.
- Después de tocar la basura.
¿Cuál es la forma correcta de lavarse las manos?
- Mójese las manos con agua corriente limpia (tibia o fría) y enjabónelas después de cerrar el grifo.
- Frote sus manos con el jabón hasta que haga espuma. Asegúrese de enjabonar las manos enteras: el dorso, entre los dedos y debajo de las uñas.
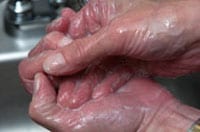 Restriegue las manos durante al menos 20 segundos. ¿Necesita un reloj? Tararee dos veces la canción del "Feliz cumpleaños" de principio a fin.
Restriegue las manos durante al menos 20 segundos. ¿Necesita un reloj? Tararee dos veces la canción del "Feliz cumpleaños" de principio a fin.- Enjuáguese bien las manos con agua corriente limpia.
- Séqueselas con una toalla limpia o al aire libre.
¿Qué debe hacer si no tiene jabón ni agua corriente limpia?
Lavarse las manos con agua y jabón es la mejor forma de reducir la cantidad de microbios en ellas en la mayoría de los casos. Si no hay agua ni jabón disponibles, use un desinfectante de manos a base de alcohol que contenga como mínimo un 60 % de alcohol. Los desinfectantes de manos a base de alcohol pueden reducir rápidamente la cantidad de microbios en las manos en algunas situaciones, pero no eliminan todos los tipos de microbios.

Es posible que los desinfectantes de manos no tengan la misma eficacia si las manos están visiblemente sucias o grasosas.
¿Cómo usar los desinfectantes de manos?
- Aplíquese el producto en la palma de una mano (lea la etiqueta para saber la cantidad correcta necesaria).
- Frótese las manos.
- Frote el producto sobre todas las superficies de las manos y los dedos hasta que estén secas.
Para obtener más información sobre el lavado de manos, visite el sitio web de los CDC sobre el lavado de manos.*Puede también llamar al 1-800-CDC-INFO o comunicarse con CDC-INFO para obtener respuestas a preguntas específicas.
* Los enlaces a sitios web pueden llevar a páginas en inglés o español.
Referencias
- globalhandwashingday.org
- Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford, JM., Jr. Water, sanitation, and hygiene interventions to reduce diarrhea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005 Jan;5(1):42-52.
- Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. The Lancet Infectious Diseases, Vol. 3, May 2003, pp 275-281. Lancet Infect Dis. 2003 May;3(5):275-81.
- WELL Fact Sheet
- UNICEF, State of the World's Children, 2008.
- Liu P, Macinga DR, Fernandez, ML, Zapka C, Hsiao H, Berger B, Arbogast JW, Moe CL. Comparison of the activity of alcohol-based handrubs against human noroviruses using the fingerpad method and quantitative real-time PCR. Food Environ Virol. 2011 Mar;3(1):35-42.
Una de las mejores formas de prevenir la propagación de infecciones y enfermedades es mantener las manos limpias. Sepa cómo y cuándo lavarse las manos.
 |
| "Mantenga la Calma y Lavese las Manos" CDC |






